Direct MCH® ~ one-step chemical hydride production using renewable energy~
Direct MCH® Process: A technology for importing large quantities of green hydrogen from overseas
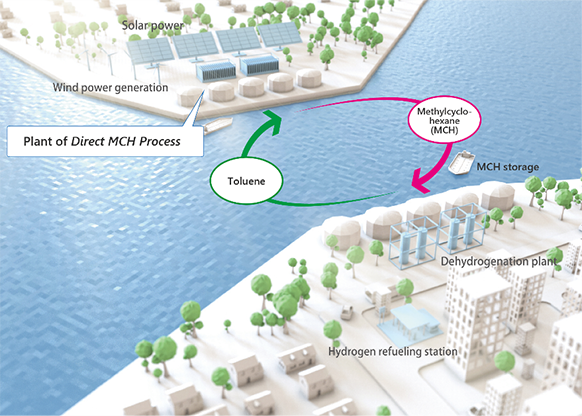
The areas that can procure large amounts of renewable energy (RE) are unevenly distributed around the world. Therefore, we are developing technologies to efficiently bring in large quantities of RE from RE-rich areas. Since renewable electricity cannot be transported over long distances, it must be converted into chemical energy such as hydrogen first. Hydrogen made from RE is called "green hydrogen" (or "CO2-free hydrogen") and the materials that carry it are called hydrogen carriers (hydrogen storage materials).
Methylcyclohexane (MCH) is a hydrogen carrier composed of liquid made by the chemical reaction of hydrogen to toluene. MCH contains over 500 times more hydrogen per volume than hydrogen gas, so it can carry hydrogen more efficiently.
In addition, MCH is a liquid with petroleum-like characteristics, so it can be used in existing petroleum infrastructure. The transported MCH can be reused as the hydrogen carrier after extracting hydrogen at demand sites such as Japan.
In the process of green hydrogen production and subsequent MCH production, cost and efficiency are the most important factors for expanding usage.
We have been developing an original process called Direct MCH® as an efficient and low-cost alternative to the conventional two-step process. Direct MCH® can produce green MCH from water, toluene, and RE in a single step without hydrogen gas by taking advantage of a unique electrochemical reaction.
Operating principle and basic research and development
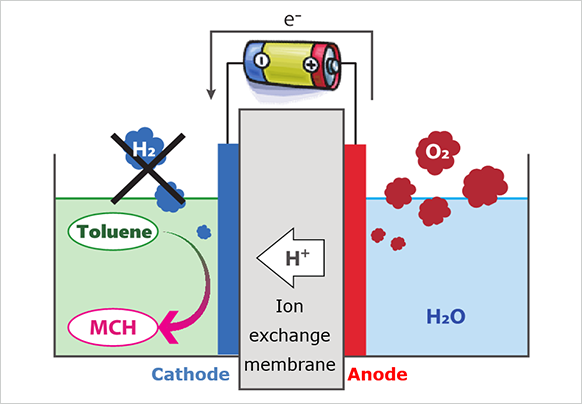
The structure of the electrolyzer, which is the reactor used in the Direct MCH® process, is shown in the figure below. In the anode, water is oxidized on the anode catalyst to produce oxygen, protons, and electrons. The resulting protons flow to the cathode through the ion exchange membrane, where they react with toluene and electrons from the external circuit on the cathode catalyst to produce MCH. The key performance indicators in this process are current density (reaction rate) and reaction selectivity (Faraday efficiency). If these performances are improved, a large amount of MCH can be produced in a small-sized electrolyzer, which means we can reduce the equipment cost and eventually achieve lower hydrogen costs.
One of the researchs and development efforts to improve such performance is focused on a discharge of the permeated water. Water is transported from anode to cathode along with protons through the membrane. When this water covers the catalyst at the cathode, the contact area between the toluene and the catalyst is reduced and the reaction selectivity for MCH production becomes lower. We are investigating ways to control the macro and micro structures and chemical characteristics in order to remove this water efficiently.
Scale-up of the electrolyzer and process development for social implementation

We have been working on the scale-up of the electrolyser and developing a process for the social implementation of Direct MCH®. We are also conducting technology demonstrations of Direct MCH® in Australia, where renewable energy is abundant.
We have successfully validated the technology for a series of green hydrogen supply chains, including green hydrogen (MCH) production in Australia using Direct MCH®, transportation to Japan, hydrogen extraction, purification, pressure boosting and filling fuel cell vehicles (FCEVs), and test driving.
(Reference: Successful demonstration of CO2-free hydrogen from Australia)
Currently, we are developing a megawatt-class electrolyzer, which will be the base for a commercial-scale plant (see the figure on the right), and developing processes for the electrolyzer plant. We are carrying out a combination of technology verifications and demonstrations in Australia in order to achieve early social implementation.
We will continue to contribute to the reduction of CO2 emissions through the establishment of a supply chain for green hydrogen.